RESEARCH
At a fundamental level, we are currently working on three different themes: the early events of metastasis; understanding the mechanisms (such as phenotypic plasticity) that allow cancer cells to tolerate chemotherapy; and developing next generation therapeutics, including nanomedicines, that can modulate the tumor stromal contexture, including the immune cells.
Cancer Immunology
We are exploring new frontiers in using the immune system to target cancer. We are studying the mechanisms underlying the interactions between cancer and immune cells. We demonstrated, for the first time, that the dual targeting of SIRP and CSF1R using a novel engineered therapeutic allows macrophages to better phagocytose cancer cells (Nature Biomedical Engineering, 2018). We were the first to design an activatable reporter nanoparticle to monitor the anti-cancer efficacy of an immune checkpoint inhibitor in real-time (PNAS 2016).Using microfluidics platforms and tumor explants, we are studying how immune cells be recruited into tumors. We are also exploring the roles of B cells in tumor immune response. Using a computational design platform, we recently designed a new linker for engineering antibody drug conjugates for treatment of cancer (Nature Biomedical Engineering, 2019).
Nanomedicine
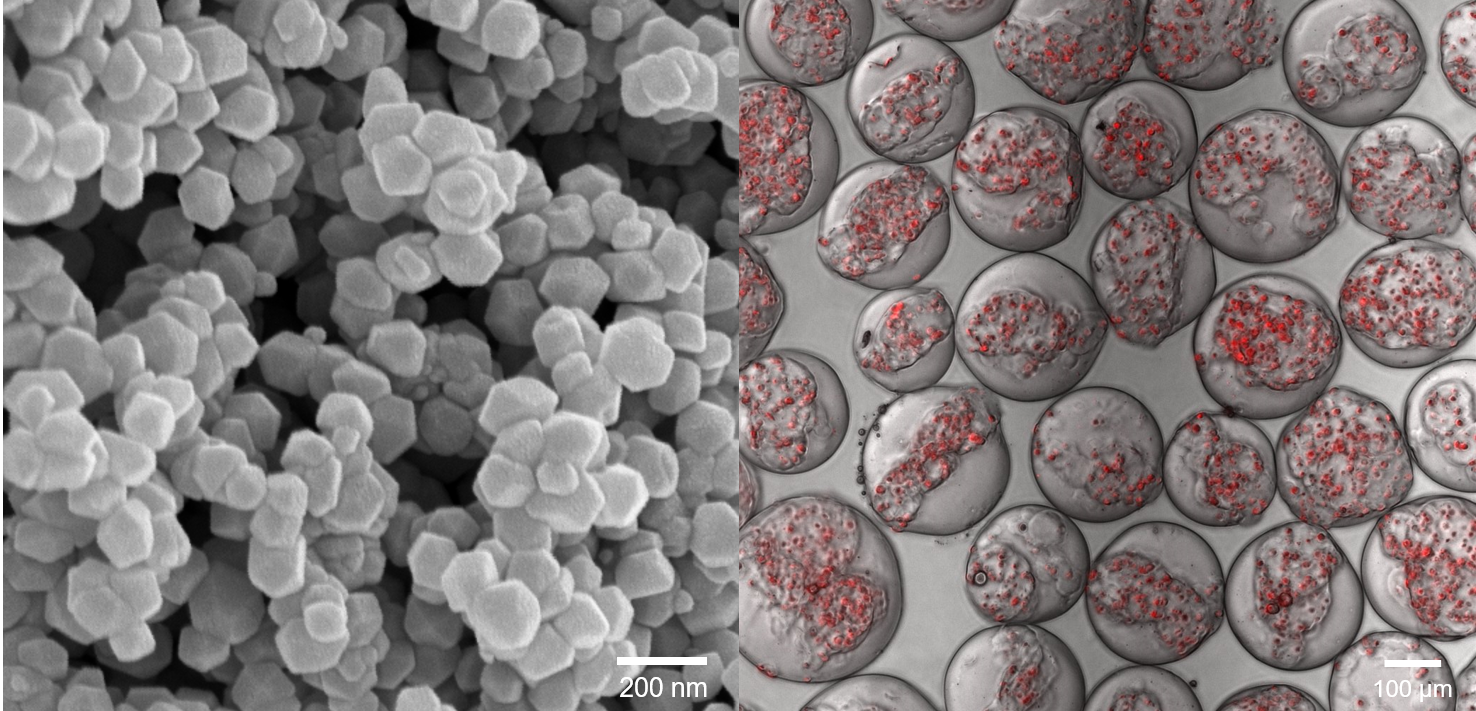
We are pioneering the use of nanotechnology to unravel new concepts in biology and medicine. We were the first to design layer by layer nanoparticles, inspired by the need to sequentially target different compartments of a tumor (Nature 2005). Using imaging techniques that allowed us to visualize nanoscale structures, we demonstrated that cancer cells can modify stromal cells in a tumor by physically communicating through tunneling nanotubes (Nature Communications, 2015). We have merged mathematical modeling and cancer biology to understand how cancer cells exhibit phenotypic plasticity and drug tolerance, and designed next generation nanomedicines to perturb these phenomenon (Nature Commun. 2015, ACS Nano 2016, Science Signaling, 2019). We are curious to explore fundamental questions around dormancy, metastasis, treatment failures, etc, using technologies at the nanoscale.
Biomaterials
We develop innovative biomaterials that can be used to treat injuries or replace damaged tissues. We have recapitulated the composition and structure of bone at the nanoscale by establishing synthetic methods for hydroxyapatite and whitlockite bone crystals, the two major bone minerals in the human body, and forming hierarchical nanochannels in bone scaffolds. For the first time, we reported a facile synthetic method of whitlockite nanoparticles and demonstrated how whitlockite has different material property from hydroxyapatite, which led whitlockite to exhibit enhanced osteogenic capacity than hydroxyapatite. Based on these nanomaterials, we have developed bone implants and spine implants that can function in vivo.
Tissue engineering and modeling/ simulation of biological phenomena
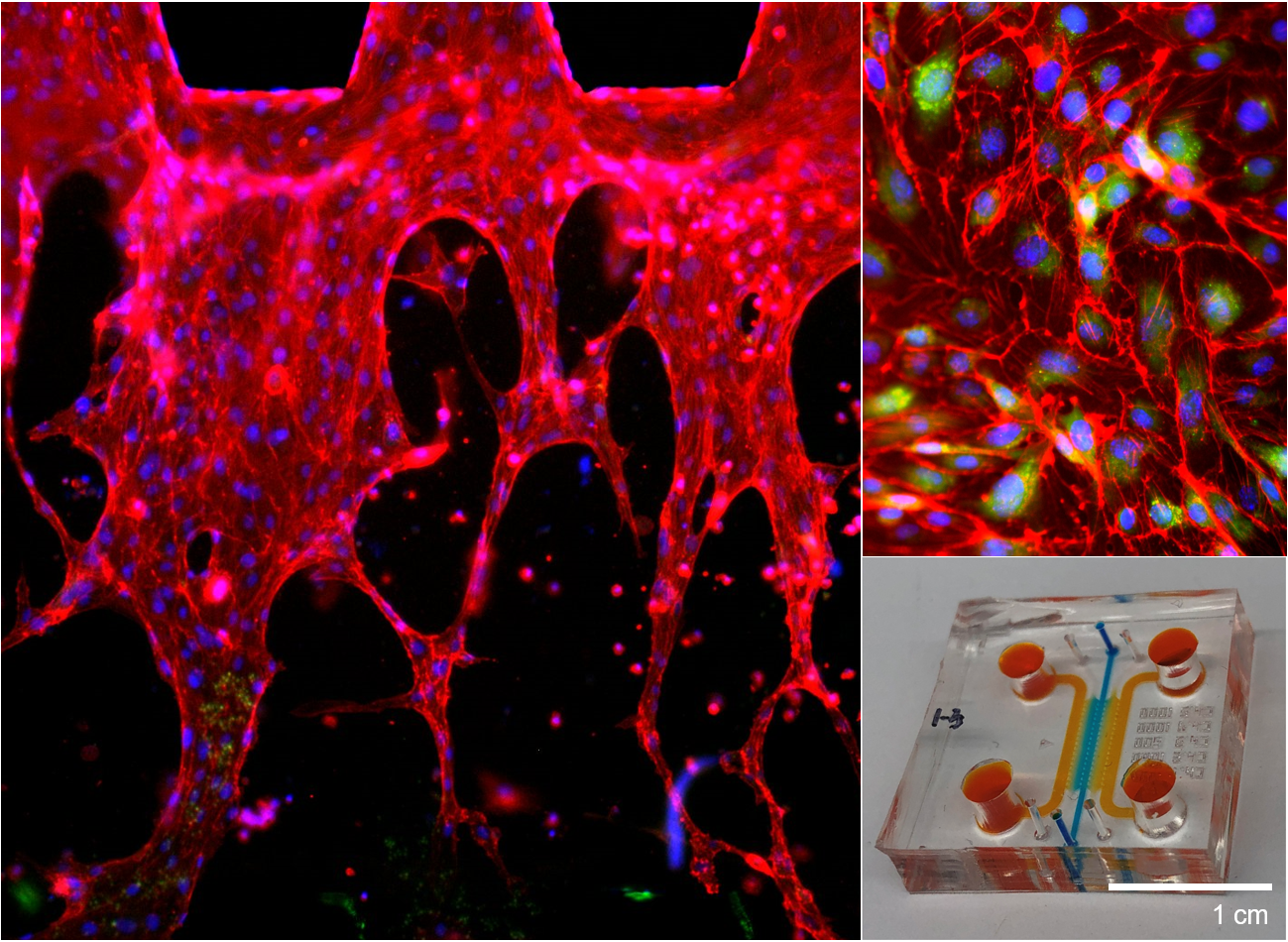
We design and tune 3D composite scaffolds to provide an optimal microenvironment for encapsulated cells to differentiate into a specific direction and develop tissue. By orchestrating different types of cells, we can control the spatial distribution of cellular structure in 3D tissue scaffold. As blood circulation is critical during tissue regeneration by supplying oxygen, nutrients, and cells, we have also successfully recapitulated perfusable microvasculature in 3D tissue scaffolds. We have engineered vascularized tissues on microfluidic chips, which can be used for testing novel drugs and investigating pathological mechanisms of various diseases, including cancer metastasis. Since each tissue dynamically interacts with other neighboring tissues, we have been using a holistic and interdisciplinary approach to engineer complex organ systems, including the usage of 3D bioprinting technology. At the same time, we regulate different types of biomaterials for directing cellular growth and differentiation as cells of each tissue type seek their niche with distinct physicochemical properties.